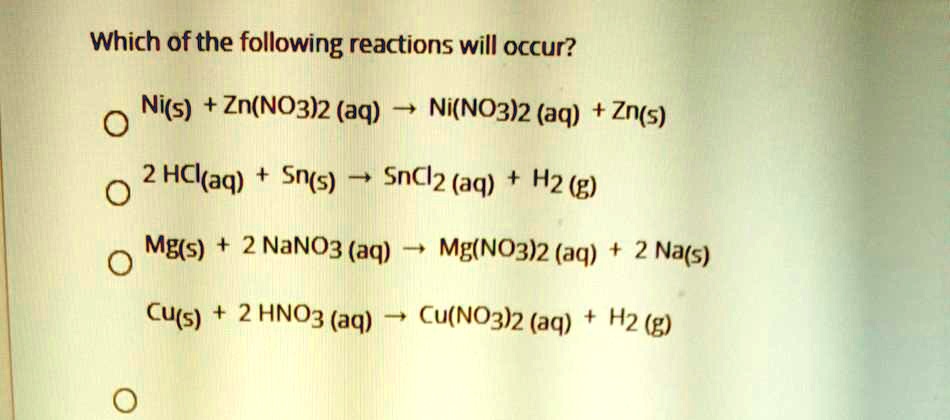Process Intensification in Nitric Acid Plants by Catalytic Oxidation of Nitric Oxide | Industrial & Engineering Chemistry Research

Dissolution of Ni deposits in concentrated HNO3 at room temperature.... | Download Scientific Diagram

Salicylic Acid Nitration by Means of Nitric Acid/Acetic Acid System: Chemical and Kinetic Characterization | Organic Process Research & Development

Selective adsorption of Pd(II) over Ag(I) in nitric acid solutions using nitrogen-donor-type adsorbents - ScienceDirect

Experiment 2, Cd test: percentages of K HNO3 of fragments of the alga... | Download Scientific Diagram

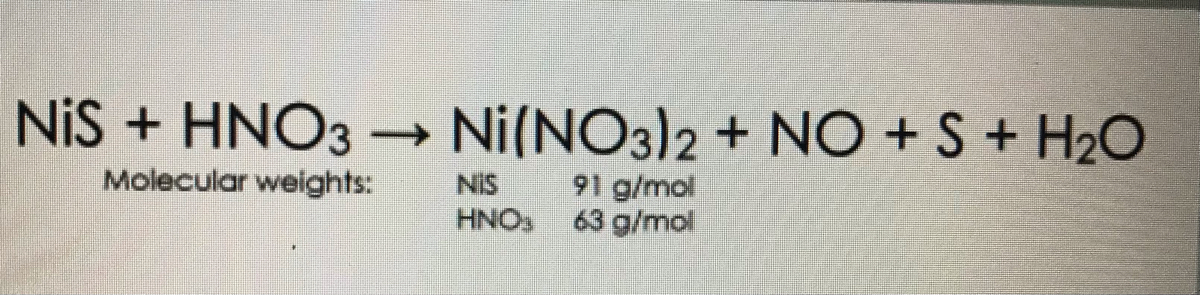
How many grams of concentrated nitric acid solution should be used to prepare 250 mL of 2.0 M HNO3? - India Site

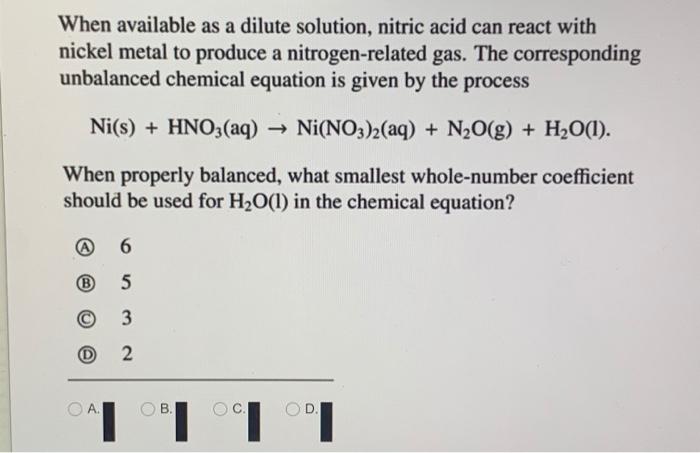
OneClass: write a balanced net ionic equation for A. dissolving of Ni (OH)2 in nitric acid. B. Ni 2+ ...

science chemistry solubility reaction nickel oxide nitric acid | Fundamental Photographs - The Art of Science
![PDF] Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability | Semantic Scholar PDF] Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/dd3b9c617b9af5ce8d4299a0c71bb33d2e456dc1/2-Table1-1.png)
PDF] Studies on Ni-Sn intermetallic compound and P-rich Ni layer at the electroless nickel UBM-solder interface and their effects on flip chip solder joint reliability | Semantic Scholar